Stem cell cultures are valuable experimental models for studying early development and disease, and they enable the generation of functional cells and tissues with therapeutic potential. However, genetic abnormalities can accumulate in stem cell lines in culture, and can be exacerbated by suboptimal culture techniques, the reprogramming process, gene editing, microbial contamination, and more. These genetic changes can have significant implications, including compromising the validity and reproducibility of research, reducing the accuracy of disease models, posing safety risks in therapeutic applications, and affecting regulatory compliance, all of which contribute to increased costs, wasted resources, and time delays.
The Importance of Karyotyping
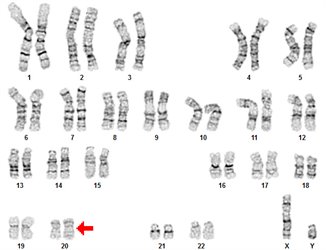
Therefore, it is important to monitor the genetic composition of a cell culture over time to help ensure the validity of any data collected. Regular characterization of stem cell lines is recommended to demonstrate culture integrity, establish baseline phenotypic profiles, and ensure the cells accurately model the target biological system1. Cytogenetic karyotyping using G-band analysis is widely recognized across the stem cell community and by regulatory bodies as the “gold standard” measurement for genetic stability. The technique provides a comprehensive visual overview of the entire chromosome complement and enables the detection of genetic abnormalities at a single cell level that can affect cellular behavior.
Importantly, stem cells intended for translational research or clinical use must meet rigorous genetic characterization release criteria for regulatory compliance. The FDA’s guidelines emphasize the importance of comprehensive cytogenetic analysis using validated methods such as karyotype analysis to ensure the safety and efficacy of stem cell products2-4. For Investigational New Drug (IND) applications and Biologics License Applications (BLA), the FDA expects detailed information on the genetic stability of the stem cell product, including data from cytogenetic testing5.
How Does Karyotyping Work?
G-banded karyotype analyses are prepared from mitotic cells arrested in metaphase when chromosomes are most condensed and visible (Figure 1). The harvest process begins with actively dividing cells, which are treated with mitotic arresting agents like colchicine, colcemid, or demecolcine to arrest cells in metaphase. During the harvest procedure, hypotonic solutions are used to increase cell volume, swelling the cells and allowing the chromosomes to spread apart when dropped on the slide, and then fixed using a methanol-acetic acid solution. Prepared slides are then stained with Leishman’s Stain. This dye binds to DNA, highlighting regions rich in adenine-thymine (A-T) pairs. Under a microscope, Leishman-banded chromosomes display unique “bar code” of light and dark bands for each chromosome. Cytogeneticists arrange these images into a karyogram to identify microscopic genetic abnormalities (>5-10Mb) including:
- Inversions
- Duplications or deletions
- Balanced and unbalanced translocations
- Aneuploidies
- >10% mosaicism6

However, G-band karyotyping also has some limitations. It requires live and actively proliferating cells, and the technique has limited resolution for detecting smaller, submicroscopic alterations (<5Mb) such as point mutations or small insertions/deletions. Moreover, the technique is labor-intensive and requires specialist skills to prepare, stain, and accurately interpret the chromosomes.
When to Karyotype?
As a quality control measure, karyotyping should be executed regularly to ensure stem cell genomic integrity and stability. Here are key instances when karyotyping should be performed1:
- Primary Donor Materials: Establish an initial genomic profile of the cell line.
- Acquisition or Derivation of a New Line: Verify cell line genetic identity to the donor material prior to starting new work.
- Initial Biobanking: Confirm the genetic identity consistency of material before establishing a master cell bank for future use or distribution.
- Start of Experimental Protocols: Confirm the genetic integrity of the cell line before beginning experiments.
- In-Process Control: Assess and monitor genomic stability at regular intervals, recommended every 10 passages of cell culture.
- Conclusion of Experiments: Confirm the genetic integrity and identity of the cell line prior to publication.
Why dPCR is not a Substitute for Karyotype Analysis
Over recent years, new techniques like digital PCR (dPCR) have been proposed as an alternative or replacement for karyotype analysis. While dPCR is faster and can detect small-scale genetic changes (i.e., point mutations, copy number variations, and low-frequency alleles) in known genetic targets, it cannot detect unknown abnormalities or identify large-scale structural abnormalities such as translocations. The two most common aberrations found in cancer cells as they become genetically unstable are aneuploidy and large-scale structural rearrangements of chromosomes7,8. For example, deletions, inversions, and translocations are commonly detected in chromosome region 9p21 in gliomas, non-small-cell lung cancers, leukemias, and melanomas9. Therefore, relying solely on dPCR means potentially missing critical genetic changes in your stem cell cultures.
Overlooking karyotyping in favor of faster and/or cheaper methods like dPCR could introduce significant risks and compromise research findings, resulting in lost time, resources and added cost in the long term. Instead, dPCR should be viewed as complementary to karyotype analysis rather than a replacement. A better strategy is to use a balanced approach that incorporates orthogonal techniques to obtain a holistic view of genetic stability.
Selecting the Right Characterization Methods
Choosing the appropriate genetic characterization methods is essential for ensuring the accuracy, safety, and regulatory compliance of stem cell cultures used in research. Researchers face a range of factors when selecting these methods, including the type of genetic abnormalities to detect, the required resolution, the specific stage of the research, and compliance with regulatory standards. This array of considerations can be overwhelming, especially given the complexity and variety of available techniques.
Karyotyping is just one part of a broader spectrum of characterization tools. Techniques such as Fluorescence In Situ Hybridization (FISH), Single Nucleotide Polymorphism (SNP) microarrays, and Short Tandem Repeat (STR) analysis each offer unique benefits and are suited to different aspects of genomic analysis.
Organizations like WiCell can help researchers navigate these options and identify the most suitable characterization program to meet their needs. WiCell offers industry-leading solutions optimized for stem cell lines, including both non-GMP and cGMP karyotype services, as well as advanced techniques such as FISH, SNP microarrays, and STR analysis. Learn more about WiCell’s comprehensive characterization capabilities and reach out to us for more information.
REFERENCES
- Ludwig TE, Andrews PW, Barbaric I, et al. ISSCR standards for the use of human stem cells in basic research. Stem Cell Reports. 2023;18(9):1744-1752. doi:10.1016/j.stemcr.2023.08.003
- Sullivan S, Stacey GN, Akazawa C, et al. Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med. 2018;13(7):859-866. doi:10.2217/rme-2018-0095
- Bauer SR. Stem Cell-based Products in Medicine: FDA Regulatory Considerations. Handbook of Stem Cells. 2004;805-814. doi:10.1016/B978-012436643-5/50163-2
- Rehakova D, Souralova T, Koutna I. Clinical-Grade Human Pluripotent Stem Cells for Cell Therapy: Characterization Strategy. Int J Mol Sci. 2020;21(7):2435. Published 2020 Mar 31. doi:10.3390/ijms21072435
- Jha BS, Farnoodian M, Bharti K. Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl Med. 2021;10(2):198-208. doi:10.1002/sctm.20-0242
- Hook EB. Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use. Am J Hum Genet. 1977;29(1):94-97.
- Thompson SL, Compton DA. Chromosomes and cancer cells. Chromosome Res. 2011;19(3):433-444. doi:10.1007/s10577-010-9179-y
- Kou F, Wu L, Ren X, Yang L. Chromosome Abnormalities: New Insights into Their Clinical Significance in Cancer. Mol Ther Oncolytics. 2020;17:562-570. Published 2020 May 26. doi:10.1016/j.omto.2020.05.010
- Cairns P, Polascik TJ, Eby Y, et al. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995;11(2):210-212. doi:10.1038/ng1095-210